Abstract
Background: Results from a randomized, Phase 3 study by the Spanish Myeloma Group (PETHEMA/GEM) previously showed that treatment with lenalidomide plus dexamethasone (Rd) may delay progression to active disease in patients (pts) with high-risk smoldering multiple myeloma (SMM), compared with observation. To further improve outcomes, addition of the anti-CD38 antibody isatuximab (Isa) to lenalidomide and dexamethasone (Isa-Rd) for the treatment of pts with high-risk SMM is being evaluated in the ongoing, randomized, multi-center, Phase 3 ITHACA study (NCT04270409). Initial findings from the safety run-in analysis of this trial have shown a manageable safety profile and encouraging, preliminary anti-myeloma activity. We now report updated safety and efficacy results from the safety run-in part of ITHACA at a median follow-up of 19.4 months.
Methods: Pts were included in the study if they had been diagnosed within 5 years with SMM (per the International Myeloma Working Group [IMWG] criteria) and had high-risk SMM according to the Mayo '20-2-20' and/or updated PETHEMA model criteria. Pts who had received prior anti-myeloma treatment were not eligible. Enrolled pts received Isa 10 mg/kg IV on day (D) 1, 8, 15, and 22 in cycle (C) 1, D1 and D15 C2-12, D1 C13-36; plus R D1-21 (25 mg C1-9; 10 mg C10-24) and d weekly (40 mg, 20 mg for ≥75 yr-old pts C1-9; 20 mg C10-24). Cycle duration was 28 days. Safety evaluations included treatment-emergent AEs (TEAEs)/serious AEs and laboratory parameters, graded by NCI-CTCAE v5.0. Response was determined by IMWG criteria (2016). Mandatory imaging by MRI and/or low-dose whole-body CT/PET-CT, and assessments of minimal residual disease (MRD, by next-generation sequencing in pts with very good partial response [VGPR] or better), were performed at protocol-defined time points. The primary study objective for the safety run-in was to confirm the recommended dose of Isa in combination with Rd. Overall response rate (ORR) and MRD negativity rate at 10-5 sensitivity were included as secondary endpoints.
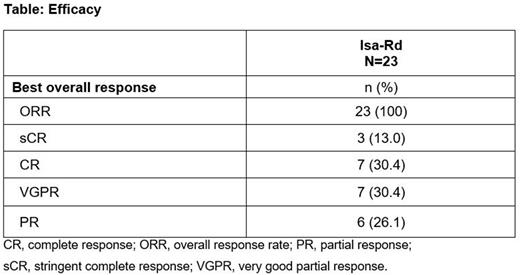
Results: At data cut-off (May 9, 2022), 23 pts (median age, 63 [range, 28-85] years) had received Isa 10 mg/kg once weekly then biweekly (QW-Q2W), in combination with Rd. Two (9%) pts met the Mayo clinical model criteria, 13 (57%) pts the updated PETHEMA model criteria and 8 (35%) pts both models' criteria for high-risk SMM. No pt had a focal lesion on MRI. The median duration of treatment exposure was 19.7 (range, 3.7-22.1) months with a median of 20 (range, 4-24) cycles. Grade ≥3 TEAEs were reported in 11 (47.8%) pts: Covid-19 pneumonia (n=3), insomnia (n=3), and pneumonia, hyperglycemia, agitation, lethargy, gastroesophageal reflux disease, retinal detachment, papular rash, and muscle spasms (n=1 each). No pt died or definitively discontinued treatment due to a TEAE. Serious TEAEs were Covid-19 pneumonia (n=3, grade ≥3), pneumonia (n=1, grade ≥3), and radicular pain, musculoskeletal chest pain, reactive arthritis, pyrexia, and amyloidosis (disease progression) (n=1 each, grade <3). The most common TEAEs (generally of grade 1-2) were insomnia (44%), constipation (30%), peripheral edema (30%), and headache (26%). Infusion reactions occurred in 2 pts (8.7%) (grade 2, infusion day 1, cycle 1). Grade ≥3 treatment-related TEAEs were reported in 9 (39.1%) pts and serious treatment-related TEAEs in 2 (8.7%) pts. Grade 3-4 neutropenia occurred in 7 (30%) pts (grade 4 in 1 [4%] pt) and grade 3 thrombocytopenia in 1 (4%) pt (no grade 4), with no treatment discontinuations due to neutropenia or thrombocytopenia. Responses deepened over time versus the initial analysis, with an ORR of 100% at a median follow-up of 19.4 (range, 18.5-19.5) months: 13.0% of pts reached a stringent complete response (sCR), 30.4% a CR, and 30.4% a VGPR. Results of the MRD assessments will be presented depending on data availability.
Conclusions: Updated results from the safety run-in part of the ITHACA trial continue to show a manageable safety profile for Isa-Rd in pts with high-risk SMM, with no definitive treatment discontinuations due to a TEAE. At a median follow-up of 19.4 months, treatment with Isa-Rd has shown promising efficacy (sCR/CR in 43.5% and ≥VGPR in 73.9% of pts), thus further confirming the recommended dose of Isa (10 mg/kg QW-Q2W) for the randomized part of the study, currently evaluating efficacy and safety of Isa-Rd vs Rd in pts with high-risk SMM.
Clinical trial registration: NCT04270409. Funding: Sanofi.
Disclosures
Mateos:Bristol Myers Squibb/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rodriguez Otero:Oncopeptides: Other: Advisory board participation ; Pfizer: Other: Advisory board participation ; Kite Pharma: Other: Advisory board participation ; AbbVie: Other: Advisory board participation ; Regeneron: Honoraria; Sanofi: Honoraria, Other: Advisory board participation; GSK: Honoraria, Other: Advisory board participation ; Amgen: Honoraria; Janssen: Honoraria, Other: Advisory board participation ; BMS-Celgene: Honoraria, Other: Advisory board participation ; Consultant in Hematology Clínica Universidad de Navarra: Current Employment; Janssen, BMS, Sanofi, Pfizer, GSK: Consultancy; Amgen, Sanofi, GSK, Janssen, BMS-Celgene, Regeneron: Speakers Bureau. Koh:Sanofi Genzyme: Research Funding. Martinez-Lopez:Bristol Myers Squibb: Consultancy, Honoraria, Other: Support for attending meetings and/or travelIncyte; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Novartis: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Roche: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Sanofi: Consultancy, Honoraria, Other: Support for attending meetings and/or travel . Parmar:Bristol Myers Squibb: Other: Advisory board participation, Research Funding; Janssen: Other: Advisory board participation; Sanofi: Other: Advisory board participation. Quach:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Hermansen:Amgen: Honoraria; Janssen-Cilag: Honoraria, Other: Advisory board participation; Bristol Myers Squibb: Honoraria; Sanofi: Honoraria; Takeda: Honoraria, Other: Conference participant support. Hungria:Bristol Myers Squibb: Honoraria; Amgen: Honoraria; GSK: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Takeda: Honoraria. Leleu:Takeda: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Pfizer: Honoraria; Amgen, Merck, BMS, GSK, Janssen, Oncopeptide, Takeda, Roche, Novartis, AbbVie, Sanofi, Gilead, Pfizer, Harpoon Therapeutic, Regeneron, Iteos: Consultancy, Honoraria; BMS: Honoraria; Janssen: Honoraria; Amgen, BMS/Celgene, Janssen, Takeda, Novartis, Sanofi, Merck, Oncopeptide, Karyopharm, Roche, Abbvie, Carsgen, GSK, and Harpoon Therapeutics: Honoraria. Schjesvold:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Skylite DX: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Targovax: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sevindik:AbbVie: Honoraria, Other: Support for attending meetings and/or travel; Amgen: Honoraria, Other: Support for attending meetings and/or travel ; Bristol Myers Squibb: Honoraria; Deva: Honoraria, Other: Support for attending meetings and/or travel; Erkim: Honoraria, Other: Support for attending meetings and/or travel; Janssen: Honoraria, Other: Support for attending meetings and/or travel; Pfizer: Honoraria; Sanofi: Honoraria; Takeda: Honoraria, Other: Support for attending meetings and/or travel. Lavrova:Excelya: Current Employment. Dubin:Sanofi: Current Employment. Devisme:Aixial: Current Employment. Lepine:Excelya: Current Employment. Macé:Sanofi: Current Employment. Morisse:Sanofi: Current Employment. Ghobrial:Amgen: Honoraria; Adaptive: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Aptitude Health: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Research Funding; GSK: Honoraria; Celgene: Research Funding; Huron Consulting: Honoraria; Menarini Silicon Biosystems: Honoraria; Oncopeptides: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria; Sognef: Honoraria; Takeda: Honoraria; The Binding Site: Honoraria; Vor Biopharma: Honoraria; Veeva Systems: Honoraria; Window Therapeutics: Other: Advisory board participation.
OffLabel Disclosure:
Investigational use of isatuximab in patients with high-risk smoldering multiple myeloma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal